Diamond physical and chemical properties
Global caracteristics
A diamond is a transparent crystal of tetrahedrally bonded carbon atoms. Diamonds have been adapted for many uses because of the material's exceptional physical characteristics. Most notable are its extreme hardness, its high dispersion index, and high thermal conductivity, with a melting point of 3820 K, which converts to 6416.33 F and 3546.85 C.
|
Chemical composition |
hardness |
density |
Refractive index |
Dispersion |
|
Carbon (C) |
10 (mohs) +/- 9000kg/mm2 |
3.515 g/cm3 +/-0.015 g/cm3 |
2.42 |
0.044 |
Hardness
Diamond is the hardest natural material that is known, its hardness set to 10, i.e. hardest, on Mohs scale of mineral hardness and having an absolute hardness value of between 90, 167, and 231 gigapascals in various tests. Diamond's hardness has been known since antiquity, and is the source of its name. However, aggregated diamond nanorods, an allotrope of carbon first synthesized in 2005, are even harder than diamond.

Mohs Scale
The hardest diamonds in the world are from the New England area in New South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is considered to be a product of the crystal growth form, which is single stage growth crystal. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice all of which affect their hardness.
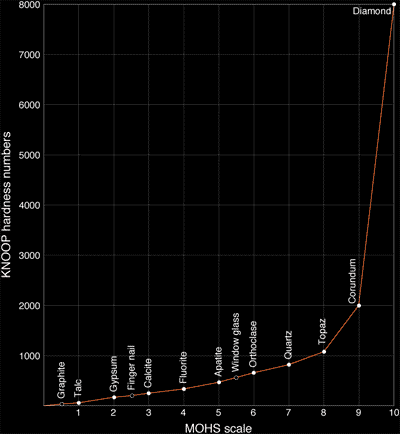
Knoop Scale
The hardness of diamonds contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well, keeping its luster over long periods of time. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in an engagement ring or wedding ring, which are often worn every day.
Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, or use of diamond powder as an abrasive. Industrial-grade diamonds are either unsuitable for use as gems or synthetically produced, which lowers their value and makes their use economically feasible. Industrial applications, especially as drill bits and engraving tools, also date to ancient times.
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent electrical insulators.
Toughness
Toughness relates to a material's ability to resist breakage from forceful impact. The toughness of natural diamond has been measured as 3.4 MN m-3/2, which is good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond is therefore more fragile in some orientations than others.












